
Colorectal Cancer
Advertisement
Sassun-Mayo N/TNM staging demonstrated superior OS stratification compared with current AJCC staging.
Dr. Eng discusses the rising global incidence of early-onset CRC, as highlighted in a recent Lancet Oncology publication.
Suvemcitug, envafolimab, and FOLFIRI may serve as a new second-line treatment option for cold tumors.
KRASG12C inhibitors can have reduced efficacy in patients with alterations in KRAS, EGFR, and other genes.
The guideline was developed using a multidisciplinary team of oncologists in collaboration with ASCO and the SUO.
ctDNA can serve as a major independent prognostic biomarker in patients with stage III colon cancer after surgery.
The combination was shown to provide a 71% response rate, pointing to a new potential treatment for this patient population.
With an OS rate of about 80%, encorafenib and cetuximab with FOLFOX may serve as a new FDA-approved frontline regimen.
Encorafenib with cetuximab was approved by the FDA in 2024 for previously treated patients with BRAF V600E-mutant mCRC.
Dr. Kopetz elaborates on the key efficacy and safety findings, including how they compare to current standard treatments.
Dr. André elaborates on the efficacy and safety profiles of both treatments and their influence on current therapies.
Dr. Fakih elaborates on the combination's objective response rate, and how it can potentially redefine treatment paradigms.
Dr. Fakih elaborates on the Fc-enhanced design of botensilimab, and how the combination compares to prior therapies.
The study showed that favezelimab/pembrolizumab did not demonstrate a survival benefit over SOC.
Dr. Weinberg provided his perspective on the CheckMate 9DW expanded analyses, as well as multiple studies in CRC.
Previously presented results of the trial showed favorable PFS rates in the m-FOLFOXIRI and cetuximab arm.
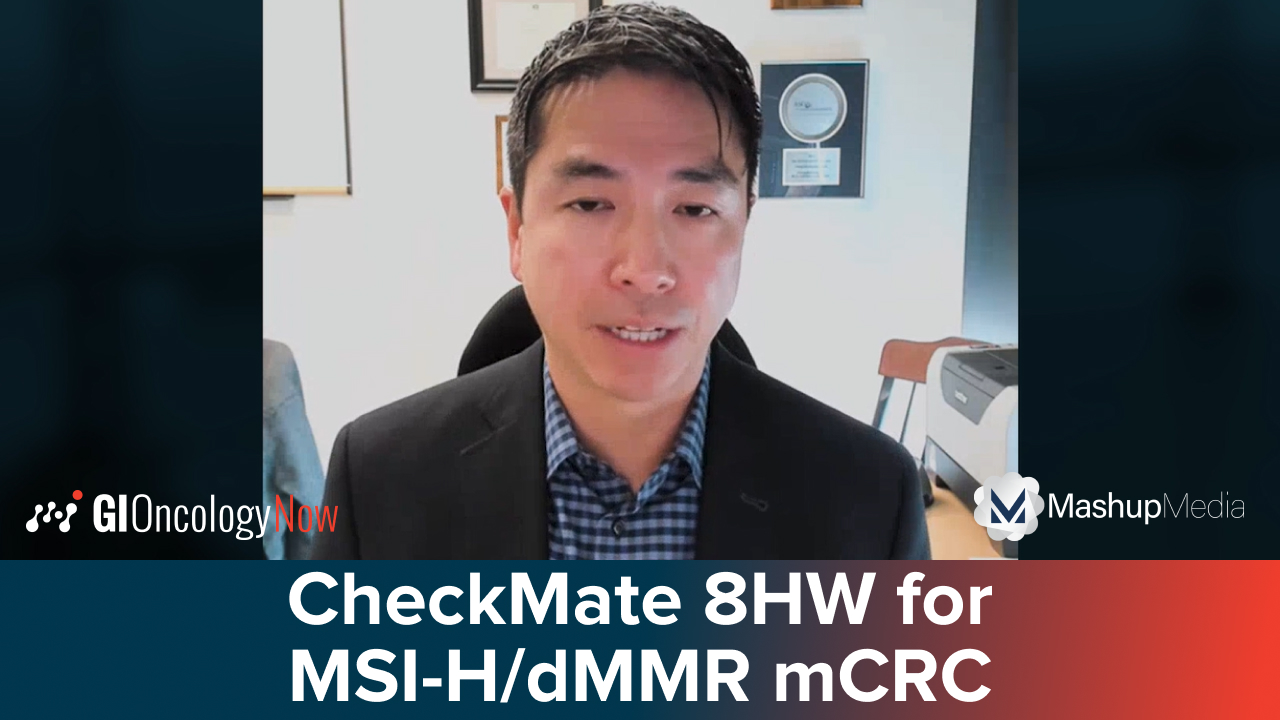
Dr. Lieu details practice-changing studies from ASCO GI 2025, including CheckMate-9DW, CheckMate 8HW, and BREAKWATER.
The study shows the prognostic value of ctDNA in detecting MRD in patients with stage II/III CRC.
The approval is based on positive results of the CodeBreaK300 trial, which demonstrated an improvement in PFS.
The FDA has approved nivo for subcutaneous injection for all solid tumors for which nivo is indicated as a monotherapy.












 © 2025 Mashup Media, LLC, a Formedics Property. All Rights Reserved.
© 2025 Mashup Media, LLC, a Formedics Property. All Rights Reserved.